Polarizability
Polarizability Assignment Help | Polarizability Homework Help
Polarizability
Anions are larger in size than cations and therefore their electron clouds are less lightly held. A small highly charged cation shall, therefore, distort the electron cloud of the large anions in a manner that it increases the electron density between the nuclei. For example, the large iodide ion by itself is perfectly symmetrical. However, when a small positively charged lithium ion comes close to the iodide ion, the anion is pulled towards the positive lithium ion. The iodide ion is said to be polarized. The polarization effect introduces covalence in LiI molecule. The power of an to distort the other ion is known as its polarizing power and the tendency of the ion to distortion is know as its polarizability. The polarizing power of a cation is proportional to is may be so marked that the bond becomes covalent. Examples of such ionic-covalent compounds include AICI3, FeCI3, SnI4 etc.
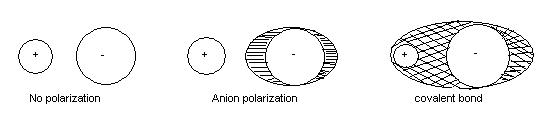
Fajan discussed the variations in non-polar character of ionic compounds in terms of polarization effects. He considered that covalent bonding was an extreme example of polarization between two atoms where maximum electron density is between the two nuclei.
Fajan proposed the following rules for the prediction of non-polar character between two ions.
(i) high charge of the cation or anion.
(ii) small size of positive ion.
(iii) large size of negative ion.
For more help in Polarizability click the button below to submit your homework assignment
Fajan discussed the variations in non-polar character of ionic compounds in terms of polarization effects. He considered that covalent bonding was an extreme example of polarization between two atoms where maximum electron density is between the two nuclei.
Fajan proposed the following rules for the prediction of non-polar character between two ions.
(i) high charge of the cation or anion.
(ii) small size of positive ion.
(iii) large size of negative ion.
(i) High charge of cation or anion.
The highly charged cation will exert more polarization on the electron density of the anion and will introduce more covalence in its compounds. On the other hand, the highly charged anion will get more readily polarized. Therefore, the polarizing power of a cation and polarizability of an anion will increase with increase in the charge on the ions.(ii) Small size of cation.
The small sized cation will have high charge density and therefore it will be able to distort (polarize) the electron cloud of the anion more effectively.(iii) Large size of anion.
The polarizability (to be able to get distorted) of an anion increases with increase in its size because its electron charge cloud is not firmly held by its own nuclear charge an can be thus readily polarized by incoming cations.For more help in Polarizability click the button below to submit your homework assignment